
PPID (Pituitary Pars Intermedia Dysfunction)
PPID (formerly known as Equine Cushing’s Disease) is the result of a progressive degeneration of hypothalamic dopaminergic neurons, resulting in hyperplasia and adenoma formation in the pars intermedia of the pituitary gland and an excessive production of adrenocorticotropic hormone (ACTH).1
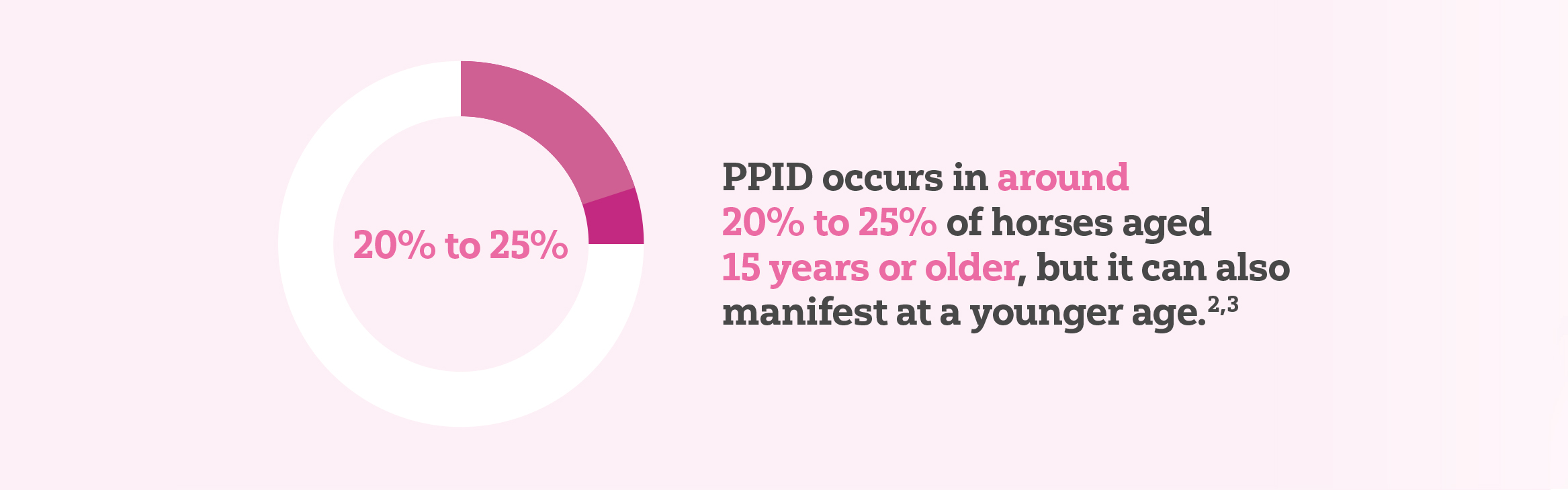
PPID is relatively uncommon in horses less than 15 years of age but prevalence is high in elderly horses.1
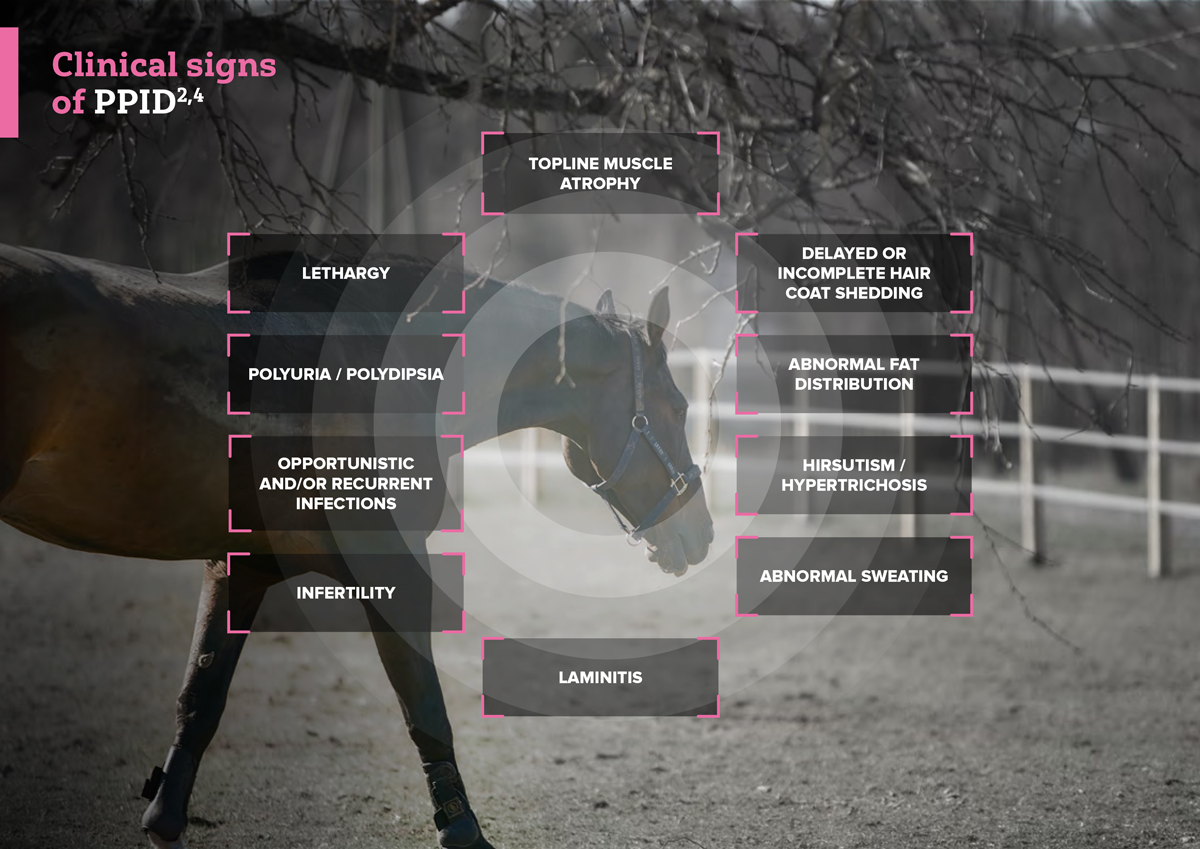
Diagnosis of PPID
PPID should be diagnosed based on clinical signs and diagnostic testing.1 The recommended first-line test is an ACTH baseline test.1
Early diagnosis and treatment of PPID are essential to help improve a horse’s quality of life.1,4,5
Treatment with pergolide requires a tailored approach
PPID is a life-long condition that requires ongoing, daily treatment, commonly with oral pergolide (a potent, long-acting dopamine receptor agonist) and supportive care.6 Throughout treatment, clinical responses and laboratory test results should be monitored to make any needed dose adjustments.1,5
Optimal dosing
Every horse is unique. Based on their weight and individual response to therapy, the dose of pergolide should be adjusted to achieve an optimal treatment response. Regular assessment of the dose is recommended (every 4-6 weeks until stabilisation or improvement of clinical signs) to achieve the best treatment outcome with the least side effects.1,5
Convenient administration
Owners need to administer pergolide orally to their horse on a daily basis and adjust doses when required. Therefore, pergolide treatment should be simple, allowing for accurate dose titration and minimising risk to the administrator of accidental exposure.
Pergocoat, the pergolide treatment for every unique horse
Pergocoat is indicated for symptomatic treatment of clinical signs associated with PPID.

For a bespoke pergolide treatment to suit every horse’s needs, prescribe Pergocoat
Optimal dosing without the need to split tablets
Recommended starting dose of Pergocoat, based on the horse’s body weight:
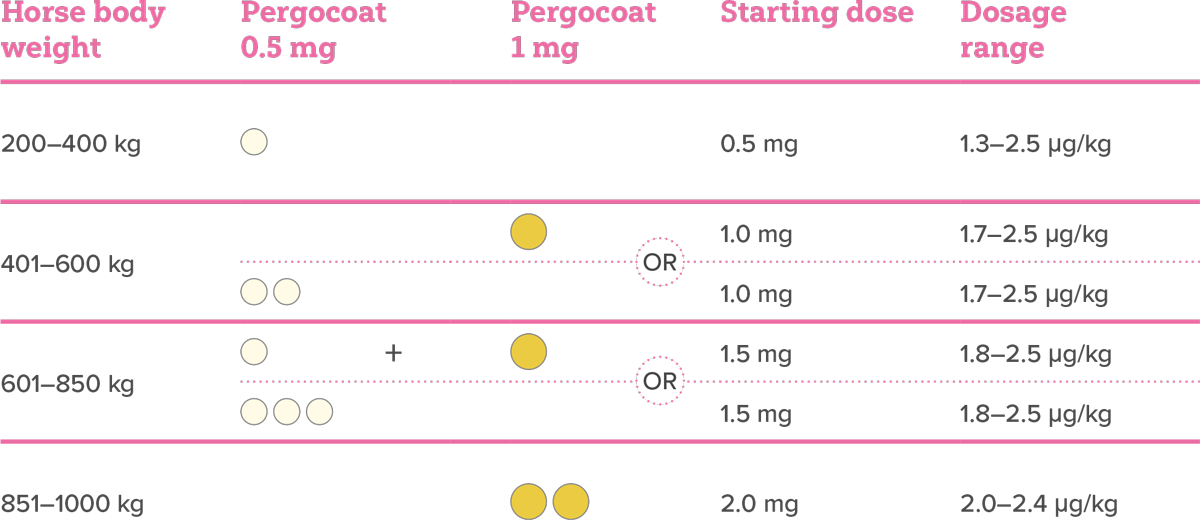
Treatment monitoring and dose optimisation should be performed every 4-6 weeks, until stabilisation or improvement of clinical signs is observed.

For further information contact: Dechra, Sansaw Business Park, Hadnall, Shrewsbury, Shropshire SY4 4AS. T +44 (0)1939 211200 F +44 (0)1939 211201 www.dechra.co.uk.
Registered Office: 24 Cheshire Avenue, Cheshire Business Park, Lostock Gralam, Northwich CW9 7UA. Registered in England and Wales, Company Registration No.5385888.
Dechra Veterinary Products Limited is a trading business of Dechra Pharmaceuticals Limited. Pergocoat contains Pergolide 0.5 / 1.0 mg per tablet UK POM-V Use medicines responsibly: www.noah.co.uk/responsible. DVP1496. April 2024.

Leave a Reply