The list of endocrine diseases reported in companion animals is extensive. Endocrine diseases are extremely common in ferrets, with insulinomas (benign, insulin-producing tumours causing hypoglycaemia) and hyperadrenocorticism (caused by increased production of androgen and oestrogen by adrenal gland tumours) being most prevalent.
In contrast, in rabbits, endocrine diseases are considered relatively rare (either truly uncommon or underdiagnosed) and have most often been reported in literature as either an incidental finding during postmortem exams or in laboratory animals.
However, despite the lack of extensive information, they should never be ruled out when compiling a differential diagnosis list. In particular, few cases of adrenal endocrine disease have been reported in rabbits. This article will provide information regarding this uncommon disease.
Anatomy
The left adrenal gland lies lateral to the aorta and cranial to the left renal vein. The right gland is more cranial, and located dorsolateral to the caudal vena cava, caudal to the liver and cranial to the right renal vein. This anatomical location is similar in all small exotic mammal species, but adrenals tend to be larger in smaller species (Reese, 2011).
The left cranial and left medial adrenal arteries from the aorta supply the left adrenal gland. The right cranial and right caudal adrenal arteries from the abdominal aorta and the right renal artery supply the right adrenal gland (Popesko et al, 1992).
Kigata and Shibata (2018) reported the number of adrenal arteries varied from 3 to 16 on the right, 3 to 18 on the left, and 9 to 30 in total in each individual. These findings demonstrate the remarkable individual variation in arterial supply to the rabbit adrenal gland, suggesting such variations should always be considered during treatments in the rabbit.
Adrenocortical disease
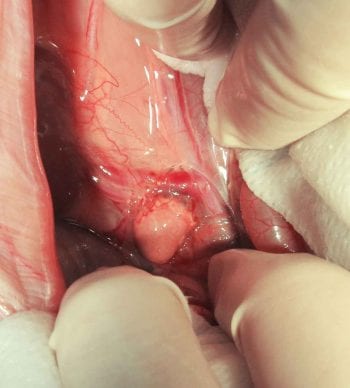
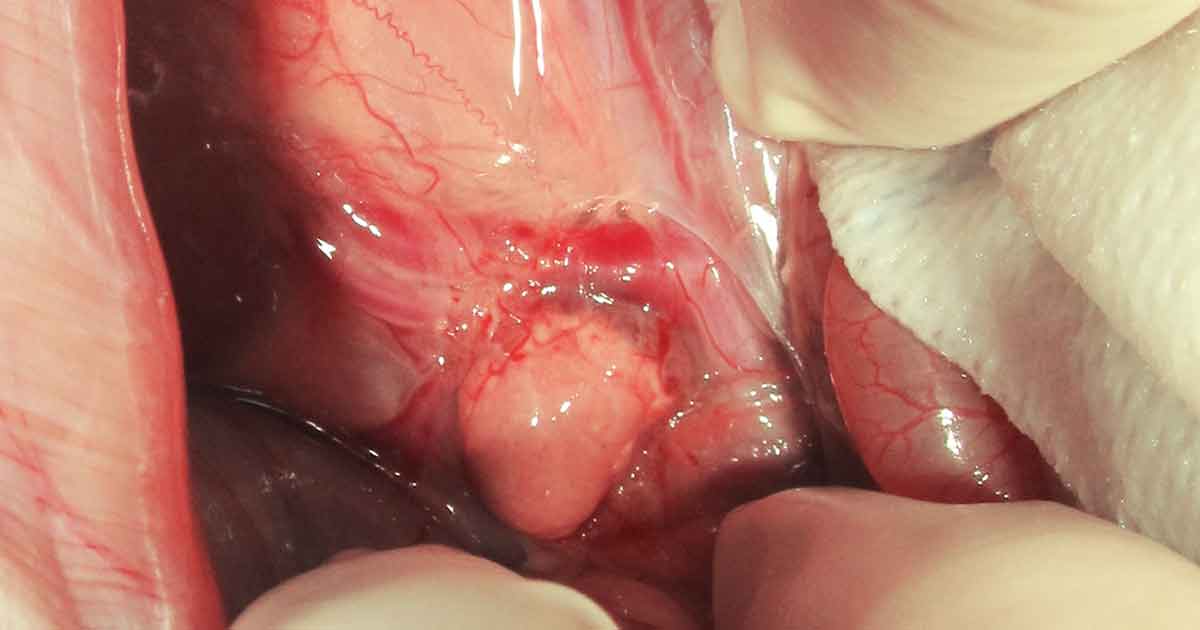
adrenal gland during exploratory laparotomy.
Adrenocortical disease is well described in ferrets and linked to early gonadectomy (Rosenthal and Wyre, 2012). Neutered ferrets produce sex hormones from the adrenal gland. Over time, without negative feedback, the adrenal gland becomes hyperplastic and potentially neoplastic.
Both hyperplasia and neoplasia can be functional diseases, and treatment options reported over the years in this species include surgery, melatonin, gonadotropin-releasing hormone (GnRH) agonists and, to some extent, medications targeting specific hormones (Lennox, 2013a).
Androgen-producing adrenal tumours have been described in several species, including humans (Lennox and Chitty, 2006). Reports exist in rabbits, but little information is available on this subject in this species (Lennox and Chitty, 2006; Varga, 2011; Lennox, 2013b; Baine et al, 2014; Lennox and Fecteau, 2014; Rose et al, 2016) and adrenal tumours seems to be rarely diagnosed (Banzato et al, 2015).
The pathogenesis of this disease in rabbits is not well understood, but some hypothesise a similar mechanism to ferrets and mice in which gonadectomy contributes to adrenocortical hyperplasia and later tumorigenesis (Bielinska et al, 2006; Beuschlein et al, 2012).
Beyer et al (1969) suggested adrenal sex hormone production in normal ovariectomised rabbits, further supporting this hypothesis. Juvenile female rabbits after ovariectomy demonstrated continued sexual behaviours, which were then significantly reduced after adrenalectomy. In contrast, ovariectomy eliminated all sexual behaviours in rats (Beyer et al, 2007).
In the few reported cases of adrenal gland hyperplasia and neoplasia in rabbits, age of onset was between 6 and 10 years, with average age at diagnosis of 7.6 years. Males and females were affected, although males were overrepresented. Unusual and persistent sexual and aggressive behaviour in neutered – especially older – animals are some of the common presenting signs, linked to increased testosterone levels.
Owners may report their rabbit mounting and chasing humans and other pets or objects, biting, scratching, urine spraying, enlarged clitoris in females, decreased appetite and muscle loss (Lennox, 2013a, 2013b; Baine et al, 2014).
In male rabbits, cryptorchidism, where neutering history is not available, should be considered as a differential in cases with increased libido and should, therefore, be ruled out (for example, abdominal ultrasound and measurement of testosterone levels). Additional possible causes for elevated sex hormones in a castrated animal include gonadal remnant, ectopic gonadal tissue or other neoplasia.
Human chorionic gonadotropin stimulation testing has been used in rabbit patients to determine if an ovarian remnant or ectopic gonadal tissue is present (Fecteau et al, 2007; Hoffman et al, 2009). In the reported cases, the right or left adrenal gland were affected by hyperplasia or neoplasia (for example, adenoma or carcinoma).
The diagnosis was mainly based on a combination of history, presenting signs and endocrinological essay. Elevated testosterone levels and absence of other obvious testosterone sources were demonstrated. In two cases, elevated progestin concentration were also seen (Lennox and Fecteau, 2014; Blaine et al, 2014).
In fact, early evidence exists that other hormones, including progesterone, may also be elevated. Beyer et al (1969) established that, in normal rabbits, the adrenal glands secrete appreciable amounts of sex steroids.
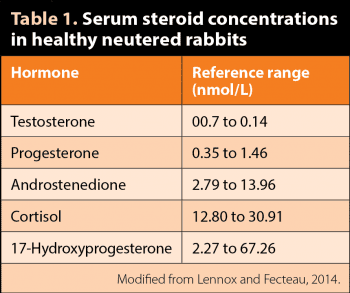
Most mammals also have accessory adrenal-cortical tissue nodules and sex steroids may also be produced from other non-glandular tissues. Fecteau et al (2007) reported reference values for serum steroid concentrations in healthy neutered rabbits of both sexes. Testosterone values ranged from 0.07nmol/L to 0.14 nmol/L (Table 1). It should be noted different reference intervals have been established for rabbits by other laboratories (Goericke-Pesch et al, 2015).
A study found the mean testosterone level in seven male juvenile New Zealand white rabbits was more than six times higher 90 days after gonadectomy (Goericke-Pesch et al, 2015) compared to values obtained in the laboratory used in a 2016 case report (Rose et al, 2016). These discrepancies – which may result from use of different methods, different rabbit populations and husbandry techniques – may complicate the diagnosis in practice.
Often, different studies may also use different units, and this may need to be taken into consideration. Confirmation of the diagnostic suspicion is achieved by histopathology after surgical removal of the gland or postmortem examination.
Diagnostic imaging
The adrenal glands cannot be seen in radiographic images of small exotic mammals (Reese and Hein, 2011). It is much easier, due to their size, to see these glands using ultrasonography.
In healthy rabbits, the adrenals have an ovoid to almost round shape. They appear hypoechoic to the surrounding tissues, with an evident hyperechoic line in the middle, but not a distinct corticomedullary junction (Banzato et al, 2015).
Reese (2011) reported instead a marked distinction between the hypoechoic cortex and the echoneutral medulla. Ultrasonographic length and diameter of rabbits’ adrenal glands have been reported in rabbits (Reese, 2011; Banzato et al, 2015; Table 2). It is important to remember the reference adrenal sizes were obtained in mixed-breed rabbits, and the published intervals may not be representative of the adrenal size of larger rabbits.
Ultrasonography – considered a useful diagnostic test to detect adrenal masses in other small mammals (Besso et al, 2000) – is reported to have variable usefulness for a definitive diagnosis in rabbits (Lennox, 2013b; Rose et al, 2016). Banzato et al (2015) also report a positive correlation between body size and adrenal gland length, which may explain these findings.
Furthermore, adrenal gland size in wild rabbits has been documented to change with seasons (for example, peaks in size are seen during the summer months; Myers, 1967).
Treatment
Possible treatment modalities proposed for hormone-secreting adrenocortical tumours have included medical options and surgical adrenalectomy. Medical options for adrenal gland disease depend on the part of the adrenal cortex affected (for example, zona reticularis secreting gonadotropins, zona fasciculata secreting glucocorticoids or zona glomerulosa secreting mineralcorticoids).
In rabbits, only two tumours affecting the zona reticularis have been confirmed (Lennox and Chitty, 2006; Baine, 2014). In ferrets, melatonin, GnRH agonists (such as leuprorelin acetate and deslorelin) and a GnRH vaccine have been successfully used for lesions affecting the zona reticularis (Rosenthal and Wyre, 2012; Miller et al, 2013).
In female rabbits, administration of slow-release deslorelin implants resulted in reversible suppression of ovarian function that lasted until the implants were removed after approximately nine months (Geyer, 2015).
In another study, application of a slow-release GnRH agonist implant did not reduce testosterone concentrations nor affected spermatogenesis in treated New Zealand white rabbits. Authors concluded such an implant did not induce hormonal castration in male rabbits over a period of 90 days, indicating it may not be a suitable alternative to surgical castration in this species (Goericke-Pesch et al, 2015).
In a rabbit with adrenal cortical hyperplasia, leuprorelin acetate was given at 100µg/kg to 200µg/kg IM every two weeks and caused only short-term improvement of clinical signs, but ultimately, adrenalectomy was required for cessation of clinical signs (Lennox and Chitty, 2006).

However, clinical signs recurred three months later and use of finasteride was unsuccessful. In another case, deslorelin implant placement resulted in partial resolution of clinical signs and decrease of testosterone level three months later (Rose et al, 2016). However, testosterone level did not normalise.
Other hormonal therapies attempted in some of the cases reported in the literature – alone or in combination with surgery – included flutamide and trilostane. The response was variable. More research would be needed to evaluate the efficacy and safety of these therapies in rabbits.
Surgical adrenalectomy is considered the preferred treatment for humans, dogs and cats with resectable androgen-secreting tumours, whereas placement of a deslorelin implant is reported to yield a longer mean time without clinical signs of hyperadrenocorticism than surgical adrenalectomy in ferrets (Lennox and Wagner, 2012).
Response to adrenalectomy in rabbits is variable. In some of the reported cases (three out of six) that underwent successful partial or total adrenalectomy, surgery led to only temporary resolution of clinical signs (Lennox, 2013b).
The author also performed one total left adrenalectomy, which resulted in only partial resolution of clinical signs lasting for several months till the rabbit died for unrelated reasons. It is possible recurrence of clinical signs in all these cases is associated with testosterone production by the remaining gland secondary to hyperplasia, neoplasia or metastasis.
The technique for adrenalectomy is reportedly similar to that described for ferrets (Lennox, 2013b). Isolation and ligation of supplying vessels followed by blunt dissection can allow complete excision of the left gland.
Right adrenalectomy is more challenging due to close association of the gland with the vena cava. In ferrets, evidence exists that partial occlusion of the vena cava by an adrenal tumour promotes the formation of collateral circulation via the vertebral venous sinus and the azygous vein. Therefore, resection of the adrenal gland and associated vena cava may not result in severe circulatory disturbances or death (Lennox, 2013b).
No information exists on similar development of collateral circulation in the rabbit and ligation of the vena cava cannot, therefore, be recommended in this species (Lennox, 2013b).
Conclusions
Additional research is certainly needed to elucidate the pathophysiology of the disease in rabbits. The prognosis for adrenal gland disease in rabbits remains uncertain due to the low number of cases so far reported. For the same reason, specific indications on the best approach to follow cannot be given.
Please refer to the literature provided for more information on this subject. Drugs indicated may be used under the cascade.