
In his recent webinar, A New Approach to Canine OA Management, Duncan Lascelles, Professor of Small Animal Surgery and Pain Management at North Carolina State University, describes a new conceptual approach and therapeutic for the management of canine OA pain.
Early treatment matters in OA pain

As part of what he describes as the new approach to OA, Prof Lascelles explains why we need to be identifying, diagnosing and treating OA-associated pain in younger dogs.
Because OA is an age-related problem in humans, we often transpose that to dogs. However, the most common cause of OA in dogs is actually developmental disease, meaning dogs can be affected from a young age and throughout their life1. Pain is present from the early stages and is “central to the deterioration we see associated with canine osteoarthritis”. Through wind-up and ongoing sensitisation, pain severely impacts dogs’ quality of life and accelerates disease progression1,2.
Tackling OA pain at the first opportunity offers the best chance of disrupting this cycle of progressive damage2. “The sooner you can effect effective early pain relief, then the sooner you can prevent that downward spiral,” explains Prof Lascelles.
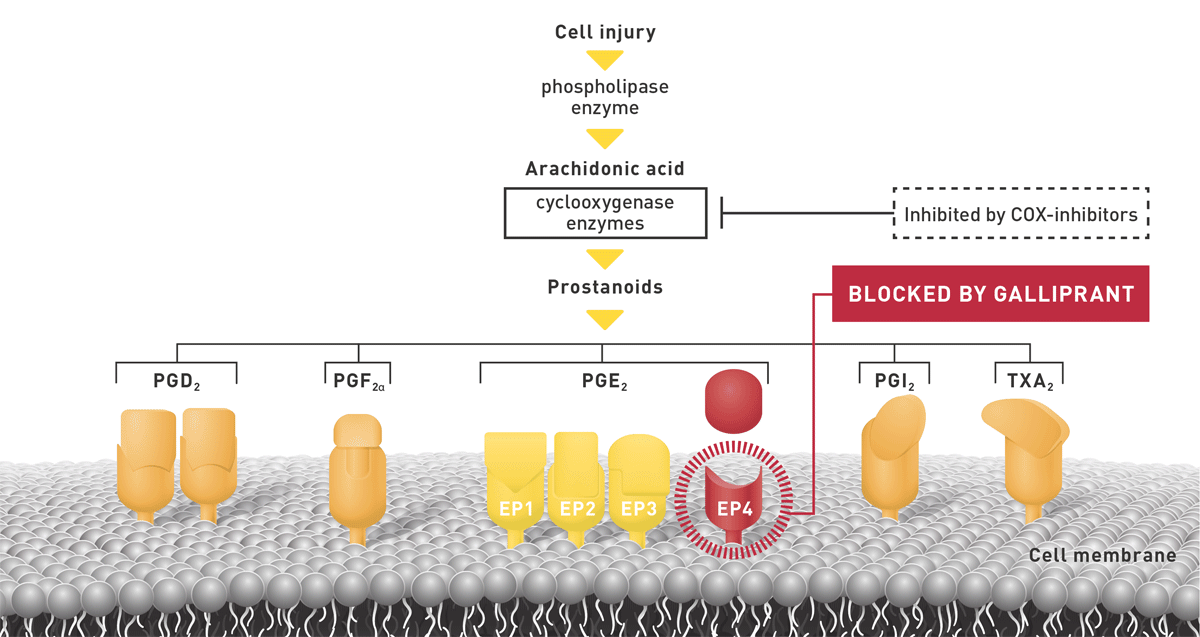
However, owner concerns about prolonged treatment sometimes delay pain relief3. While traditional COX-inhibiting NSAIDs have been a cornerstone of OA pain relief for decades, our understanding of the pain pathway has evolved4,5. We now know that prostaglandins not only have a role in pain and inflammation, but they are also important for maintaining homeostasis and functional balance within the gastrointestinal and renal systems5.
A new targeted approach to OA from first diagnosis
Prof Lascelles also discusses a new, first-in-class treatment: Galliprant™ (grapiprant), which is now available for the targeted treatment of OA pain5,6*. With its new mode of action, Galliprant can be used from first diagnosis and for as long as needed.†
- Galliprant specifically targets EP4, the key receptor for OA pain and inflammation5. By specifically targeting EP4, Galliprant does not inhibit the COX enzymes, minimising the impact on gastrointestinal and kidney homeostasis4,5.
- The safety profile was investigated in the target animal safety study, which showed that Galliprant was well-tolerated in healthy dogs over nine months at doses equivalent to 15 times the target dose7. No treatment was stopped or dogs removed from the study due to adverse events7.
- Galliprant delivers proven pain relief, with success seen by owners and confirmed by vets6.
- Treatment with Galliprant showed statistically significant improvements in pain severity and interference with daily activities vs placebo at days 7, 14, 21 and 28 (P≤0.05 for all)6.
Watch the mode of action video and see how you can get on top of OA pain* at Galliprant.co.uk
To be the first to hear Galliprant news, and for more resources, sign up to MyElanco.co.uk
* Indication: Galliprant is indicated for the treatment of pain associated with mild to moderate osteoarthritis in dogs.
† As field studies were limited to 28 days, longer-term treatment should be considered carefully and regular monitoring undertaken by the vet.
Grapiprant is a non-steroidal, non-cyclooxygenase inhibiting, anti-inflammatory drug in the piprant class. It is a selective antagonist of the EP4 receptor. Galliprant tablets contain the active substance grapiprant. Legal category POM-V (UK). Further information is available from the Summary of Product Characteristics or datasheet. Information on this veterinary medicinal product is available on the website of the European Medicines Agency http://www.ema.europa.eu/. For further information call Elanco Animal Health on +44 (0)1256 353131 or write to: Elanco Animal Health, Lilly House, Priestley Road, Basingstoke, Hampshire RG24 9NL, United Kingdom.
Use medicines responsibly www.noah.co.uk/responsible (UK).
Galliprant, Elanco and the diagonal bar logo are trademarks of Elanco or its affiliates.
©2019 Elanco or its affiliates. Date of preparation: May 2019. PM-UK-19-0118.

Leave a Reply