Cats and dogs may be infected with vector-borne pathogens – many of which can cause disease and contribute to existing clinical syndromes. Rapid diagnosis is important for correct effective treatment to be initiated and screening for subclinical infections to be carried out. This is becoming increasingly important, as a wide range of exotic vector-borne pathogens are being seen in imported and travelled dogs and cats.
While highly sensitive specific serological and PCR tests are available for many of these parasites, blood smear examination remains a useful initial screening tool. Although sensitivity of blood smear examination can be low for many vector-borne pathogens found in blood, specificity is high, and smears can be prepared and examined rapidly.
Appropriate treatment and control measures can then immediately be put in place. It is also a very cheap way of identifying multiple parasites in the infected patient. Blood smears, therefore, remain an important part of the armoury in diagnosing vector-borne pathogens.
Examination of blood smears by light microscopy forms a vital part of the diagnosis of vector-borne parasites with life stages found in the blood stream.
The use of blood smears as a diagnostic tool can be easily overlooked when other diagnostic tools, such as serology and PCR, have been developed to be widely commercially available, and are highly sensitive and specific. They are also, however, sometimes expensive and some take time for results to become available.
It is a mistake to view blood smears and these other diagnostic tests as mutually exclusive. Blood smears are a relatively insensitive diagnostic tool compared with these techniques and a negative blood smear cannot be used to rule out any parasitic infection. They are, however, extremely useful as an initial screening tool, with results almost immediately available with a high test specificity in experienced hands. They enable a range of parasites to be detected by a single test and are very inexpensive.
This broad diagnostic approach and speed of diagnosis is invaluable in the acutely ill patient, but also in pets imported from abroad. In these animals, it is desirable to test for a wide range of parasites, but costs may be prohibitive. Examination of blood smears in-house is a useful skill as results can be obtained very rapidly and some parasites seen in blood smears, such as Hepatozoon species and trypanosomes, are relatively large and easy to identify. Others, however, such as Mycoplasma haemofelis, are very small and may be missed or misidentified as stain artefact. It is, therefore, vitally important blood smears are also sent to an external laboratory for examination unless a very clear diagnosis presents itself from in-house examination.
Sending a freshly prepared blood smear with an ethylenediamine tetra-acetic acid (EDTA) sample ensures some interpretation can be made by the laboratory even if their EDTA sample is clotted. It is still very useful, however, to develop the skill of preparing and examining blood films in-house, so clear diagnoses can be made early and acted on, as well as confirming the quality of the blood sample.
Collection and handling of blood samples for smear preparation
Correct blood collection and handling is critical to avoid morphological artefacts, and parasite and cell degradation. Poor sample quality is likely to reduce both the diagnostic sensitivity and specificity of subsequent smears. The sample taker should do the following:
- Use the jugular vein, if possible, as it allows rapid collection without repeated suction – reducing the risk of clot formation and haemolysis.
- Avoid fine needles (at least 21 gauge in dogs and 23 gauge in cats) and remove the needle before filling the tube to reduce haemolysis.
- Avoid sampling through an IV cannula through which fluids are administered as this can result in sample dilution.
- Avoid overfilling the EDTA tube above the line as it promotes clot formation.
- Mix the sample by gentle inversion rather than shaking, as shaking will induce haemolysis.
- Make fresh smears as soon as possible after collection as changes in cell morphology can occur with sample storage. Red cells show increased susceptibility to Iysis after just 24 hours in EDTA. Platelets have short lifespans and tend to clump over time, making the detection of indwelling parasite life stages, such as those of Anaplasma platys, potentially more difficult to detect. Parasites such as Mycoplasma species will also quickly detach themselves from infected cells unless smears are made quickly.
- Mix the sample several times immediately before a portion is removed for testing, while avoiding excessive mixing to prevent physical trauma to cells.
Preparing a blood smear
Blood smears are quick and simple to prepare, but require practice to perform with the correct motion – leading to an even film. They can be prepared in the following way:
- A small drop of blood is placed on the slide using a capillary tube.
- A second slide (the spreader slide) is placed on to the slide holding the blood drop at approximately a 30° angle.
- The spreader slide is moved backwards until it comes into contact with the blood drop, which then spreads along the edge of the slide.
- The spreader slide is then moved forward smoothly without breaking contact with the sample slide.
- As the movement reaches the end of the slide, a feathered edge should naturally be created if the action has been smooth and the drop of blood not too large.
- The slide should then be air dried, fixed in methanol for three minutes and then stained either with Diff-Quik, Wright’s stain or Giemsa stain. If samples are not going to be prepared immediately, or are to be sent to an external laboratory for blood smear preparation, then blood samples can be stored in EDTA tubes. Alternatively, samples can be prepared and air dried without fixing and staining before sending to an external laboratory.
Blood film examination
Cells will be less densely packed on the slide at the feathered edge, where they should be single layered or at the long edge of the body, and it is in these locations that examination should take place. A ×10 objective lens can be used for initial scanning to locate this area near the feathered edge and then the ×40 objective lens can be used to become familiar with the cellular elements (white blood cell density, presence of large extracellular parasites such as filariae and so on), but an oil immersion ×100 objective lens is required for detailed examination and cell identification. Vector-borne parasite life stages may be seen in red or white blood cells, platelets or extracellularly.
Extracellular parasites seen
Although the majority of vector-borne parasites seen in the blood of dogs and cats are intracellular, three notable exceptions exist.
Dirofilaria species
Dirofilaria immitis is a filarial heartworm primarily of canids, but also can infect felids and ferrets. It is endemic throughout southern and central Europe, and is a significant cause of heart disease and also of bronchitis in cats. Transmission occurs through feeding by mosquitoes.
Acute clinical signs are associated with thromboembolism, subsequent pulmonary hypertension and caval syndrome. Worm death can also lead to thromboembolism and anaphylaxis. Typical resulting acute clinical signs include sudden death, anorexia, weakness, dyspnoea, vomiting and, rarely, respiratory signs linked to pleural effusion. Chronic respiratory signs tend to be more common in cats and include coughing, dyspnoea, anorexia, vomiting and, rarely, chylothorax.
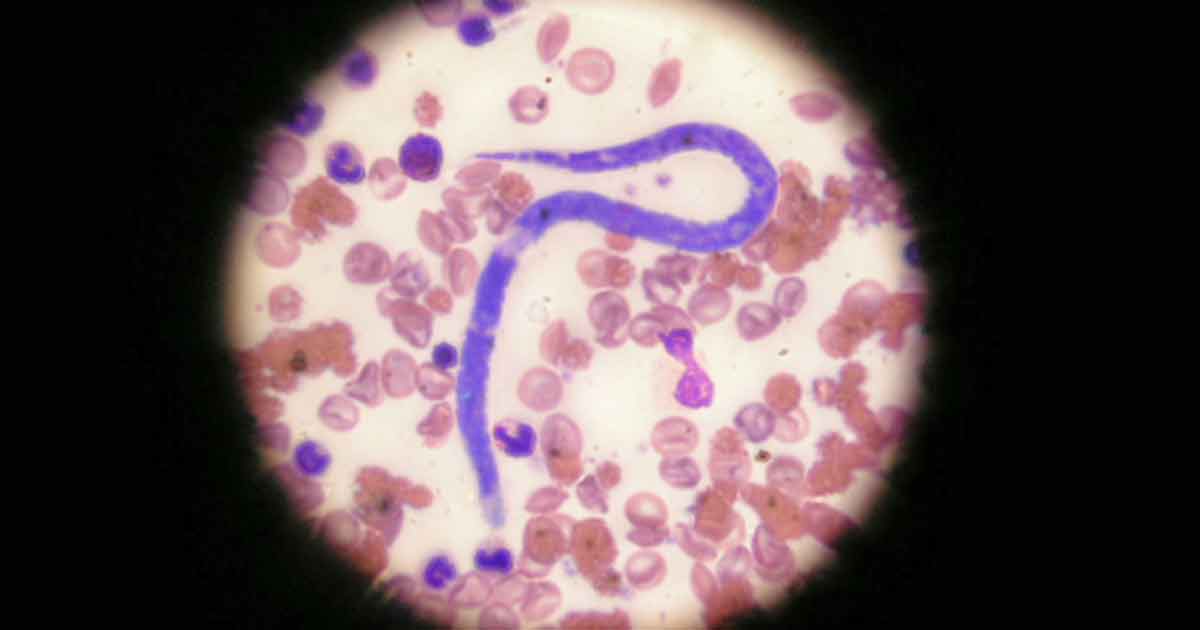
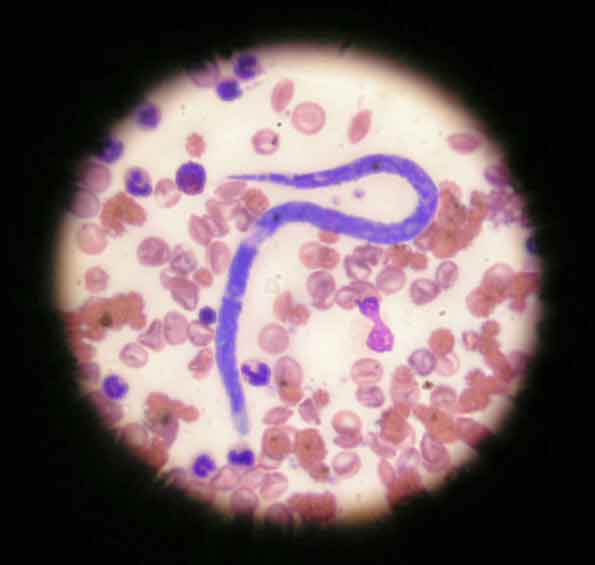
Direct smears are insensitive when looking for microfilariae (Figure 1) in the bloodstream. Concentration techniques, such as Knott’s test (a form of sedimentation test) and buffy coat examination, make direct blood examination more sensitive in canine patients, but still extremely insensitive in the cat due to microfilariae being absent or present in low numbers.
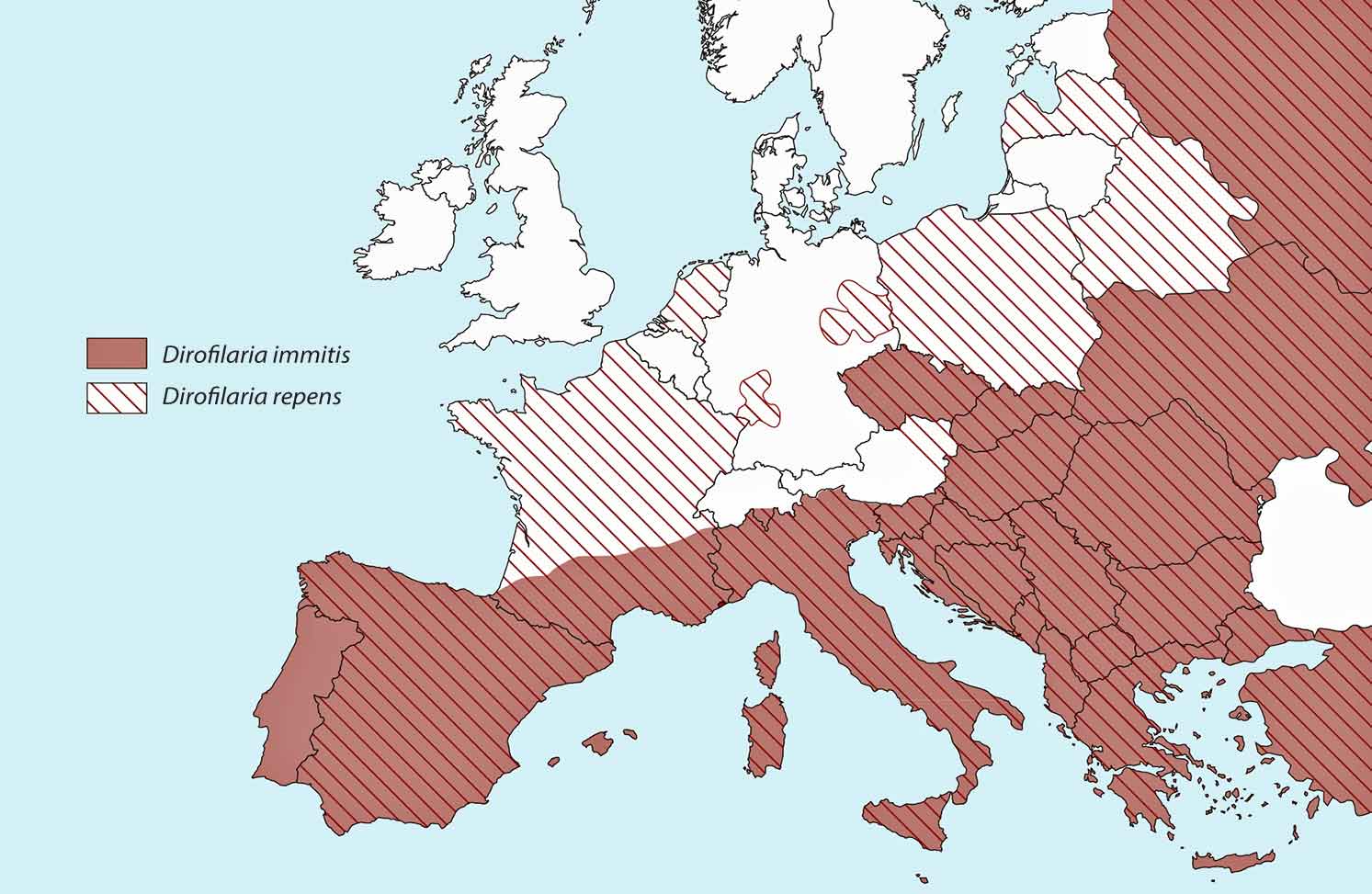
Antibody testing in cats is useful for establishing exposure to infection, and antigen blood testing in dogs remains a gold standard test. Blood smears, though, provide a means of initial screening for microfilariae of both D immitis and D repens. This is a closely related parasite present throughout eastern Europe. It is less pathogenic to cats and dogs with adult worms living in the skin, but is a zoonosis with the potential to cause creeping eruptions and conjunctivitis in people. It is capable of completing its life cycle at lower temperatures than D immitis (Morgan, 2016) and as a result, has a far wider distribution across Europe (Figure 2).
If microfilariae are detected on blood smears, the larvae of D repens must be distinguished from D immitis, which will also be present in endemic countries. Samples may be sent for microfilariae identification at an external laboratory or may be attempted in-house. The criteria on which the two species may be distinguished are summarised in Table 1.
| Table 1. Differentiating diagnostic features of Dirofilaria immitis and Dirofilaria repens microfilariae in the blood of dogs | ||
|---|---|---|
| Criteria | D immitis | D repens |
| Sheath | Missing | Missing |
| Approximate length (µm) | 205 to 283 | 260 to 308 |
| Width (µm) | 5 to 6.5 | 6 to 8 |
| Front end | Conical | Blunt |
| Posterior end | Straight | Hook shaped/bent |
Trypanosoma species.
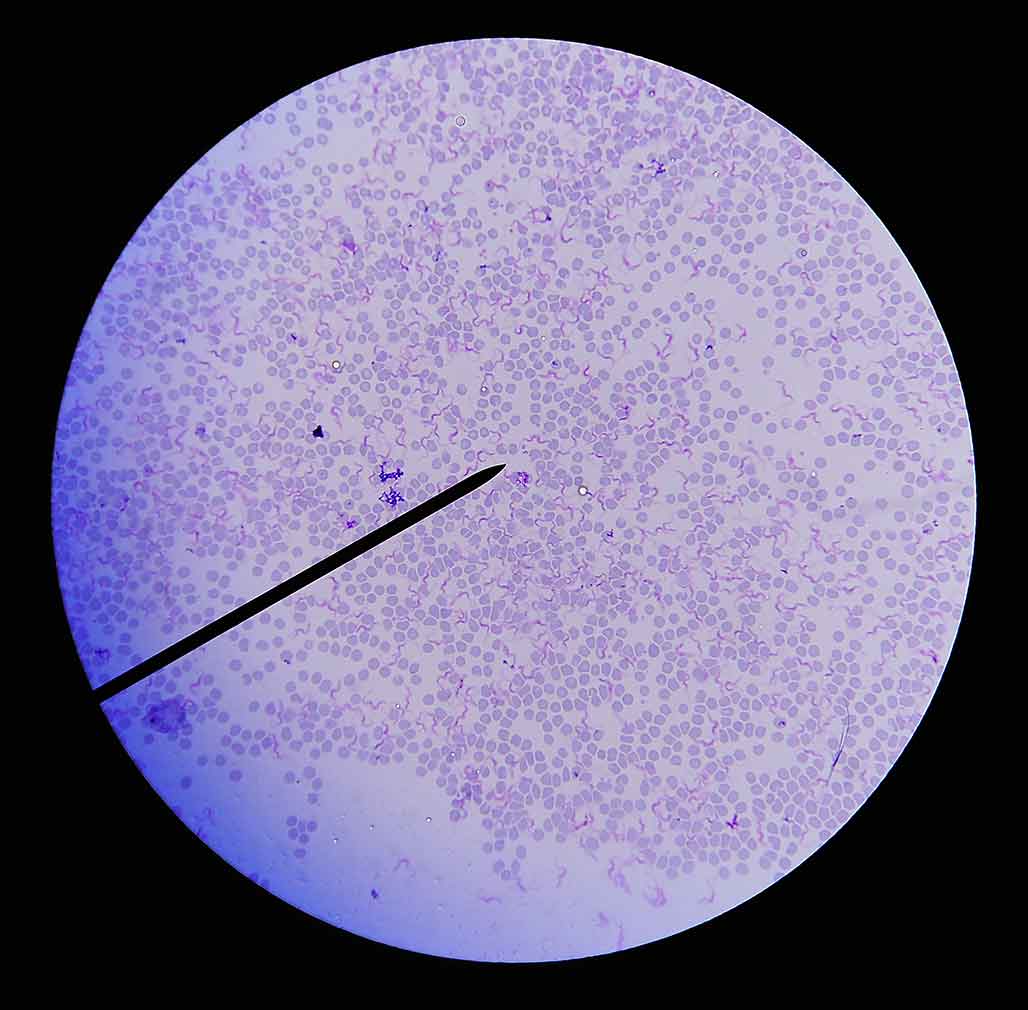
Adult rescue dogs are increasingly being imported into the UK from Africa, Asia, South America and Central America where trypanosomes, such as Trypanosma brucei and Trypanosma evansi,are endemic. These can cause acute fever, lymphadenopathy and lethargy or more chronic disease with exercise intolerance, cardiopathies and anorexia, often leading to death without treatment. Blood smears to look for flagellate trypomastigotes (Figure 3) is a sensitive and specific means of diagnosis in clinically affected dogs where parasitaemia is often high.
Borrelia species
Borrelia burgdorferi – the cause of Lyme disease – is a tick-borne pathogen that infects dogs and, to a lesser extent, cats worldwide. Although it is a vector-borne pathogen that is initially transmitted into the bloodstream, bacteraemia is transient, and the spirochaete bacteria are rarely seen on blood films. Diagnosis is achieved through serology or PCR testing of skin lesions or the joint capsule.
Parasites of red blood cells detectable by blood smear
The most common intracellular parasites of cats and dogs seen in erythrocytes are M haemofelis in cats and Babesia species in cats and dogs.
M haemofelis
M haemofelis is a rickettsial parasite of cats, with fleas thought to play a part in its transmission. Infection can cause moderate to severe immune-mediated haemolytic anaemia and should be considered as a differential in cats presenting with this syndrome. Rickettsia, such as Haemoplasma felis, may be seen as single inclusion bodies or in chains. If these are suspected, smears should then be examined fresh and over several days to maximise the chance of detecting the organism. They should not be confused with non-parasitic inclusion bodies.
Howell-Jolly bodies are blue nuclear remnants and are a common normal finding in cats. Increased numbers are seen in splenectomised animals and in regenerative anaemia.
Heinz bodies attach to the cell membrane and, again, may be a normal finding in cats. Large numbers indicate exposure to oxidant toxins such as onions or paracetamol.
Babesia species
Babesia species are tick-borne pathogens of cats and dogs, and a cause of sometimes fatal haemolytic anaemia and thrombocytopenia. The parasites invade red blood cells and are described by their shape (piroplasms) and size in relation to the red blood cell (large or small). Several Babesia species exist in Europe that can infect dogs. The large Babesia vogeli is of relatively low pathogenicity and is transmitted by Rhipicephalus sanguineus, which is not endemic in the UK. The small Babesia microti, Babesia gibsoni and Babesia vulpes have been identified in UK Ixodes ticks and untravelled dogs.
The distribution of Babesia canis is closely linked with its tick vector, Dermacentor reticulatus. The presence of the vector in the south-west and south-east of England had raised concerns about the possibility of B canis being introduced to the UK. D reticulatus is also increasing its range and prevalence across Europe, making the introduction of B canis to the UK more likely. These concerns were realised as B canis was found to be endemic in Essex (Phipps et al, 2016) and its distribution in the UK is likely to increase with an infected tick now being found in the Welsh foci of Dermacentor ticks (de Marco et al, 2017). Feline babesiosis and the closely related highly pathogenic Cytauxzoon felis are endemic in parts of Africa and North America.
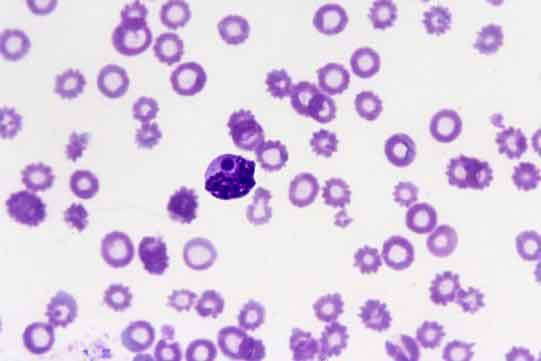
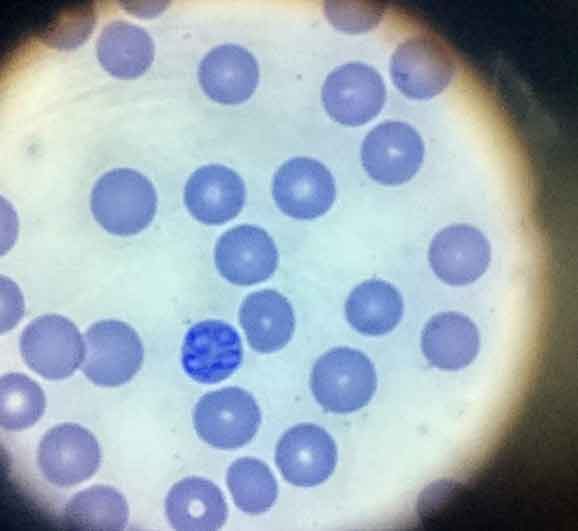
Although PCR testing is highly sensitive and specific, and allows speciation, it can take time to perform. Rapid treatment is important for prognostic outcome and peripheral blood smears are highly sensitive and specific for Babesia species diagnosis, with piroplasms (Figure 4) present in red blood cells. PCR can then be carried out if smears are negative and infection is still suspected or if speciation is required.
Parasites of white blood cells and platelets detectable by blood smear
A wide variety of vector-borne parasite life stages may be seen in white blood cells and A platys detected in platelets. The vast majority of these parasites are exotic tick-borne pathogens, including Ehrlichia canis and Hepatazoon species.
- A platys. A platys is a tick-borne pathogen of dogs thought to be transmitted by Rhipicephalus ticks. It is associated with cyclic thrombocytopenia and should be considered as a differential in dogs presenting with these signs that have visited Rhipicephalus endemic countries, including southern and eastern Europe. Serology and PCR are the most sensitive techniques for diagnosis as parasitaemia tends to be low, but a blood smear is a useful initial screen for the parasite as morulae in platelets are very characteristic (Figure 5).
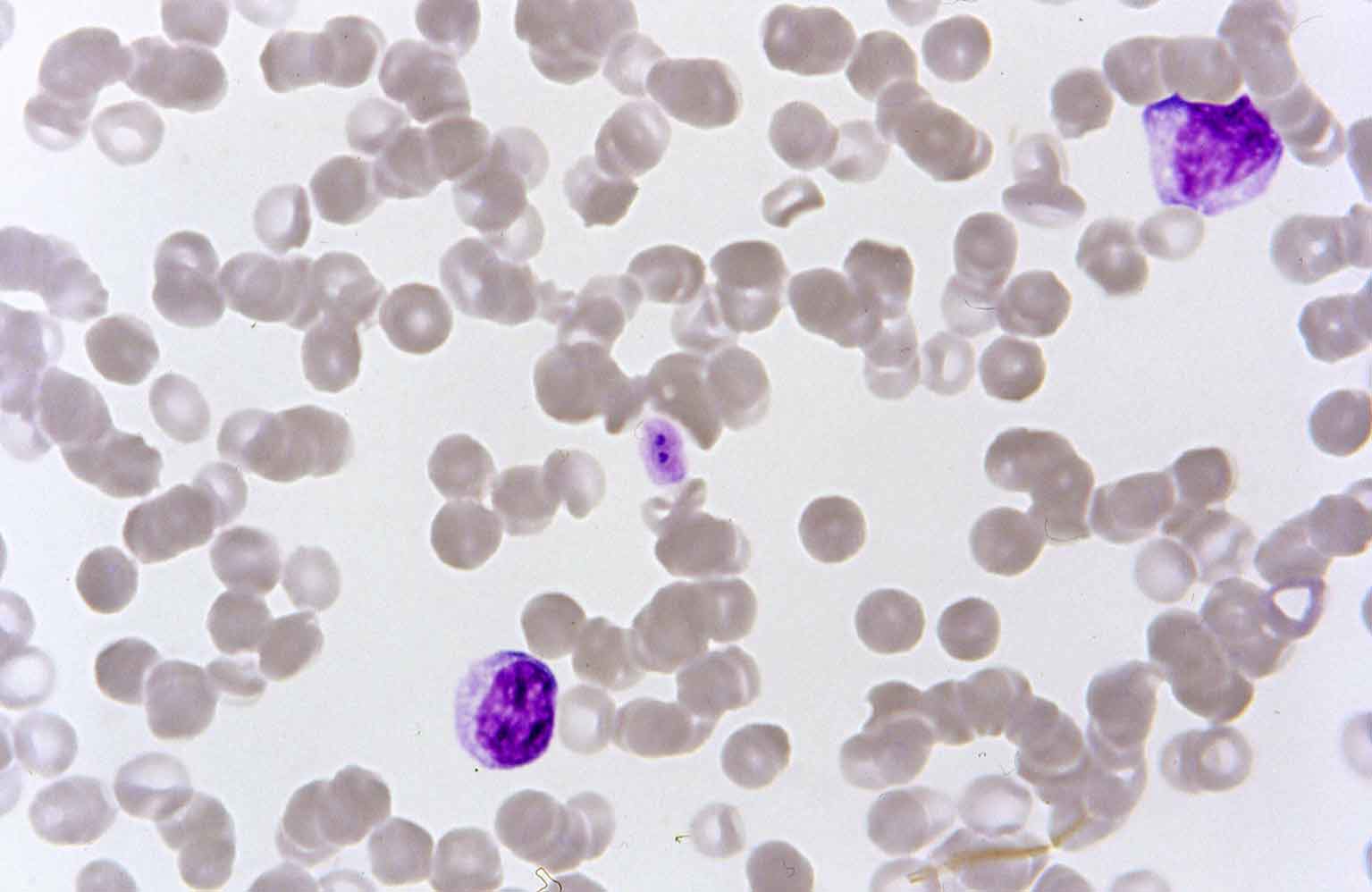
- E canis. E canis is a tick-borne pathogen primarily of dogs transmitted by Rhipicephalus ticks. It is endemic throughout southern Europe and eastern Europe, America, Africa and Asia and is being seen in increased frequency in imported dogs from abroad. It causes acute pyrexia and lymphadenopathy before either being eliminated or progressing to a carrier state for months or years before chronic ehrlichiosis develops. Dogs developing the chronic form of the disease often develop fatal complications associated with thrombocytopenia, immune suppression and anaemia. Morulae of the parasite may be detected in monocytes visualised on blood smears in approximately 4% of acute cases (Figure 6). Even in subclinical carriers, gametocytes may be detected on smears, but serology and PCR are required to rule out infection with confidence.
- Hepatozoon species. Hepatozoon canis is a canine tick-borne pathogen transmitted through ingestion of Rhipicephalus ticks. It is endemic in southern Europe and parts of Africa and Asia. Many infected dogs remain subclinical carriers, where others can develop severe, potentially life-threatening disease with lethargy, fever and emaciation. These severely affected dogs often have high levels of parasitaemia and characteristic gametocytes in monocytes are present in 0.5% to 5% of neutrophils (Figure 7). This figure can reach 100% in severe clinical cases, making blood smears a sensitive and specific diagnostic test.
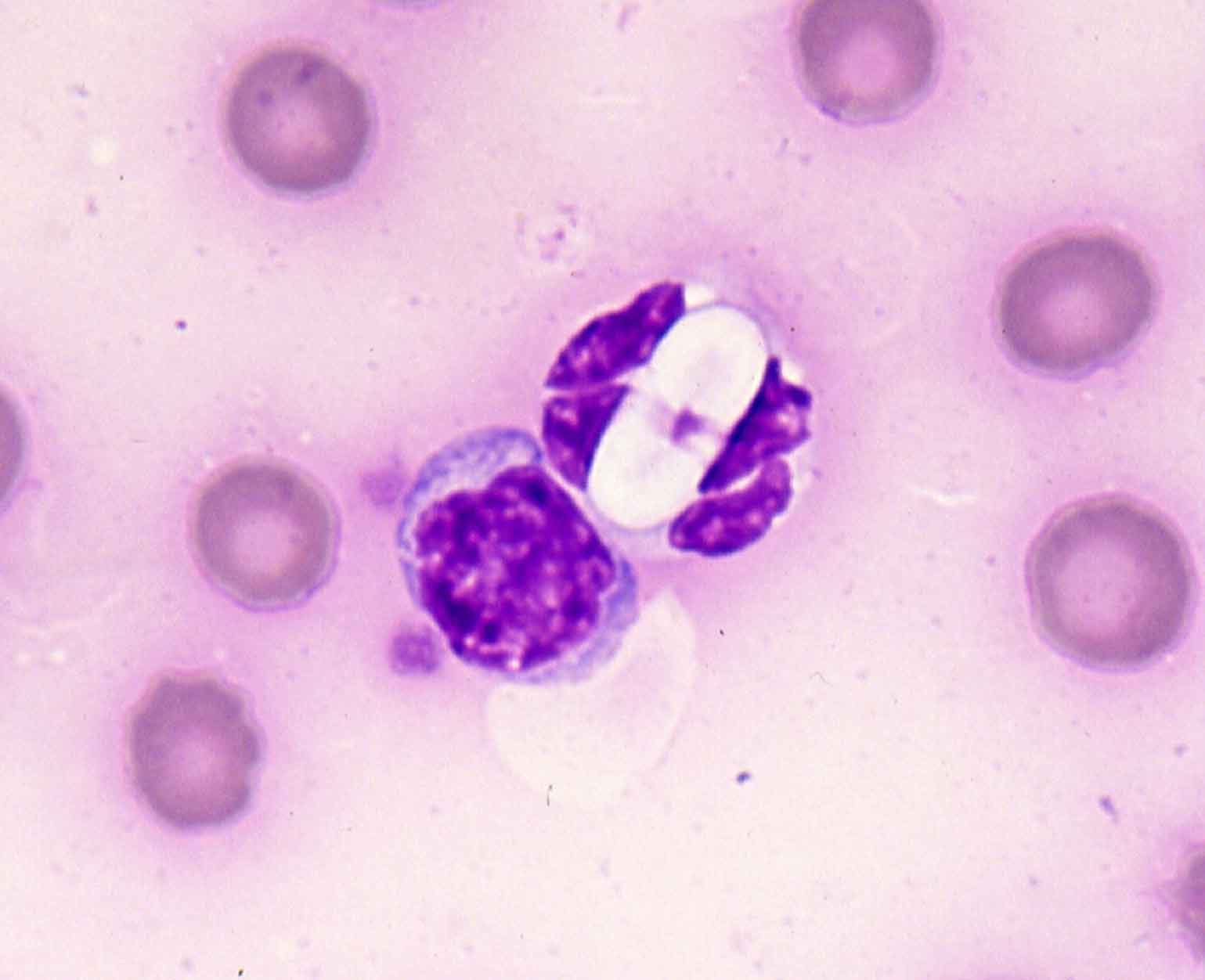
Figure 7. A Hepatazoon gametocyte. Image: Pedro Serra, NWL laboratories
Conclusion
Early diagnosis of vector-borne disease is important to initiate rapid treatment in the clinically infected patient, screen for subclinical carriers and plan for the development of chronic disease in the persistently infected patient. Awareness of the importance of diagnosing vector-borne disease is especially important in travelled and imported pets, where exotic vector-borne pathogens are likely to be encountered and present a risk to wider UK biosecurity. Blood smears remain a useful tool of initially screening for a range of these pathogens, with high specificity and at little expense.
While negative results will not confidently rule out infection for most vector-borne pathogens, identification of parasitic life stages through blood smear examination will confirm diagnosis, and allow rapid instigation of treatment and control measures. Blood smears should, therefore, remain an important tool in the diagnosis of vector-borne pathogens.