The VMD has issued a batch-specific product defect recall alert for Carprieve Small Animal Injection (Vm 02000/4229) by Norbrook Laboratories.
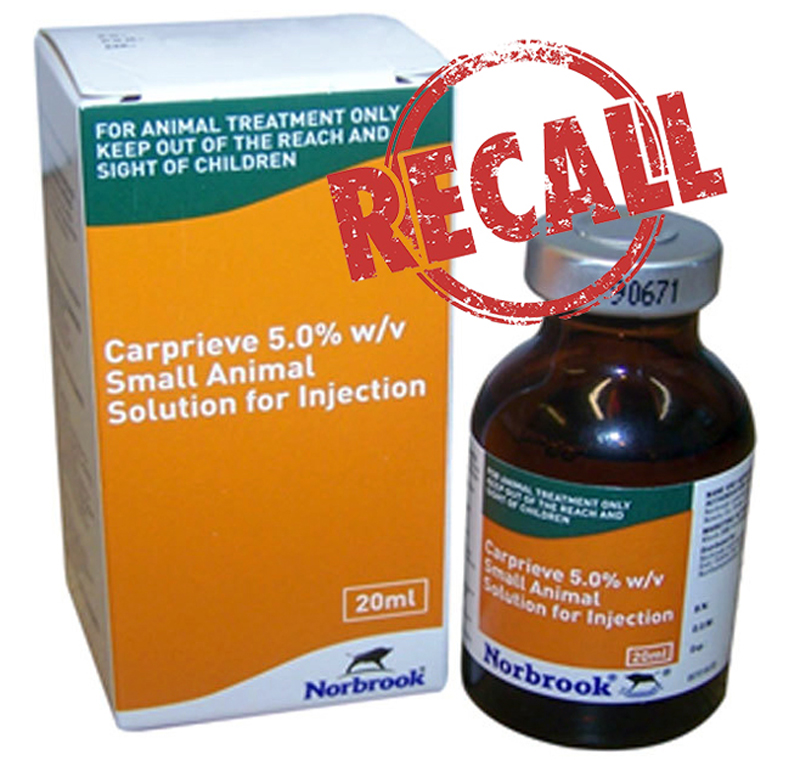 Norbrook is recalling batch 8254-90G (expiry date 21 June 2020) from the marketplace as a precautionary measure, as sterility cannot be assured.
Norbrook is recalling batch 8254-90G (expiry date 21 June 2020) from the marketplace as a precautionary measure, as sterility cannot be assured.
Contact
The company is contacting vets and wholesalers to examine inventory immediately and quarantine products subject to this recall.
For more information, telephone Naomh Thompson on 0283 026 4435 or email naomh.thompson@norbrook.co.uk
- UPDATE 6 December 2018: Norbrook has confirmed none of the batch 8254-90G (expiry date 21 June 2020) recalled by the VMD ever reached the UK market.
Leave a Reply